The U.S. Food and Drug Administration (FDA) has cleared a new rapid test from Abbott Laboratories, which the company says can detect the Coronavirus in about 5 minutes
Testing remains a crucial step in controlling the novel COVID-19 pandemic as statistics show we are losing the battle at that very stage. Continuing to supply healthcare providers with new technologies to help curb the spread of infection is right now the top most priority for public health officials and healthcare providers
 Chip Somodevilla/Getty Images
Chip Somodevilla/Getty Images
Here’s why Abbott’s announcement of receiving emergency use authorization (EUA) from the FDA is the best news for those on the front lines yet!
Until now the coronavirus strain did not really have standard testing procedures or designated test kits – there are specific labs set up for conducting tests that you may be directed to in case you feel your symptoms are specific to the coronavirus.
But even for people who are able to get tested, there can be a frustratingly long wait for results – not just hours, but often days.
 Associated Press
Associated Press
According to Narayana Health, there are five types of coronavirus tests that are currently done:
Swab Test – In this case, a special swab is used to take a sample from your nose or throat
Nasal aspirate – In this case, a saline solution will be injected into your nose and, then a sample is taken with a light suction
Tracheal aspirate – In this case, a thin tube with a torch, also known as a bronchoscope, is put into your mouth to reach your lungs from where a sample is collected.
Sputum Test – Sputum is thick mucus that gets accumulated in the lungs and comes out with a cough. During this test, you’re required to cough up sputum in a special cup or a swab is used to take a sample from your nose.
Blood test – In this case, a blood sample is taken from a vein in the arm.
 Shutterstock
Shutterstock
Here’s why it takes such a long time for results to come in according to independent, nonprofit media organization NPR
First, a sample is taken from a patient’s nose or throat, using a special swab. That swab goes into a tube and is sent to a lab. Some large hospitals have on-site molecular test labs, but most samples are sent to outside laboratories for processing. More on that later.
That transit time usually runs about 24 hours, but it could be longer, depending on how far the hospital is from the processing laboratory.
Once at the lab, the specimen is processed, which means lab workers extract the virus’s RNA, the molecule that helps regulate genes.
“That step of cleaning — the RNA extraction step — is one limiting factor,” says Cathie Klapperich, vice chair of the department of biomedical engineering at Boston University. “Only the very biggest labs have automated ways of extracting RNA from a sample and doing it quickly.”
After the RNA is extracted, technicians also must carefully mix special chemicals with each sample and run those combinations in a machine for analysis, a process called polymerase chain reaction, which can detect whether the sample is positive or negative for COVID.

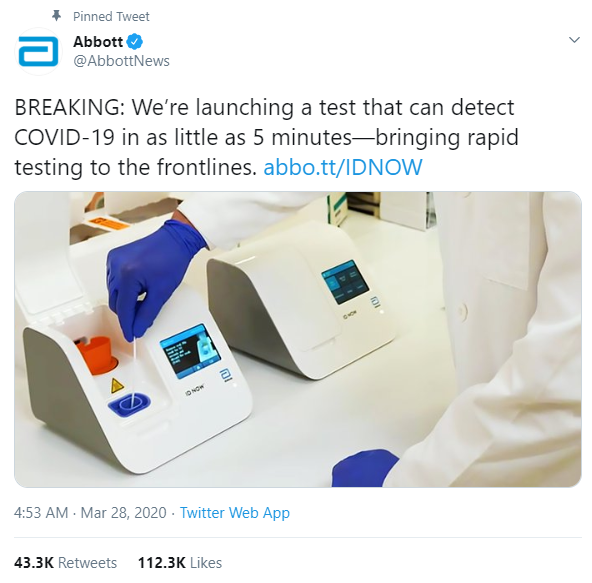
The new Abbott ID NOW COVID-19 test runs on Abbott’s ID NOWTM platform – a lightweight box (6.6 pounds and the size of a small toaster) that can sit in a variety of locations. This is the fastest available molecular point-of-care test for the detection of novel coronavirus (COVID-19), delivering positive results in as little as five minutes and negative results in 13 minutes. What makes this test so different is where it can be used: outside the four walls of a traditional hospital such as in the physicians’ office or urgent care clinics.
“The COVID-19 pandemic will be fought on multiple fronts, and a portable molecular test that offers results in minutes adds to the broad range of diagnostic solutions needed to combat this virus,” said Robert B. Ford, president and chief operating officer, Abbott. “With rapid testing on ID NOW, healthcare providers can perform molecular point-of-care testing outside the traditional four walls of a hospital in outbreak hotspots.”
Because of its small size, it can be used in more non-traditional places where people can have their results in a matter of minutes, bringing an alternate testing technology to combat the novel coronavirus.
The WHO has stressed upon testing during the outbreak, insisting containment is feasible and must remain the top priority for all countries
 Ari Jalal/Reuters
Ari Jalal/Reuters
India currently conducts about 90 tests a day, despite having the capacity for as many as 8,000 according to The Associated Press
The Government however claims it is raising the capacity of testing on a regular basis but has been criticised for not testing enough. It has one of the lowest rates in the world, with just 6.8 tests per million.
 Rajanish Kakade/AP
Rajanish Kakade/AP
Initially, India insisted on testing only those who had travelled to high-risk countries or had come in contact with an infected person or health workers treating coronavirus patients. It later said that anyone admitted to hospital with severe respiratory distress should also be tested. But with the circle of infection widening daily, the numbers are expected to grow hugely.
According to a statement from the Indian Council of Medical Research (ICMR) — the apex body in India for the formulation, coordination and promotion of biomedical research as on 27 March the total number of approved government laboratories to test for COVID-19 stand at 122 out of which 113 labs are already operational while the remaining 9 are in the process.
 PTI
PTI
Till today we have conducted 34,931 tests. Capacity utilization in the ICMR network is around 30%. We have increased some labs, 113 have been made functional & 47 private labs have been given approval, said R Ganga Ketkar of ICMR.
 Click picture for complete list of enlisted laboratories for coronavirus testing across India Shutterstock
Click picture for complete list of enlisted laboratories for coronavirus testing across India Shutterstock
However, people who need to be tested have to fit in the criteria prescribed by the ICMR. This includes symptoms that include fever, sore throat, runny nose, dyspnea, etc or individuals returned from affected countries like China, Hong Kong, Japan, South Korea, Singapore, Iran, Italy, close contact with confirmed positive cases of COVID-19 infection, all individuals evacuated and quarantined from Wuhan, China and Diamond Princess ship, Japan and other coronavirus affected countries.
India has been reluctant to expand testing, not wanting to trigger panic and overwhelm hospitals, but also because of the cost: While the tests are free for patients, they cost the government about 5,000 rupees each.
